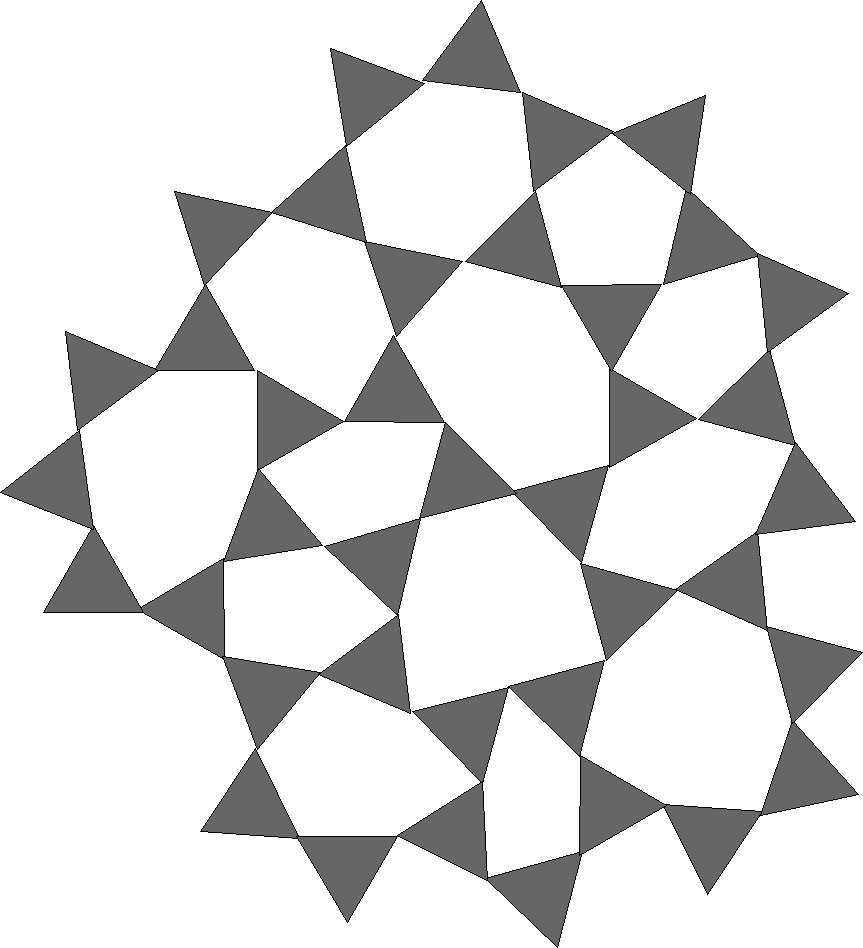
Figure 1 - Silica Glass
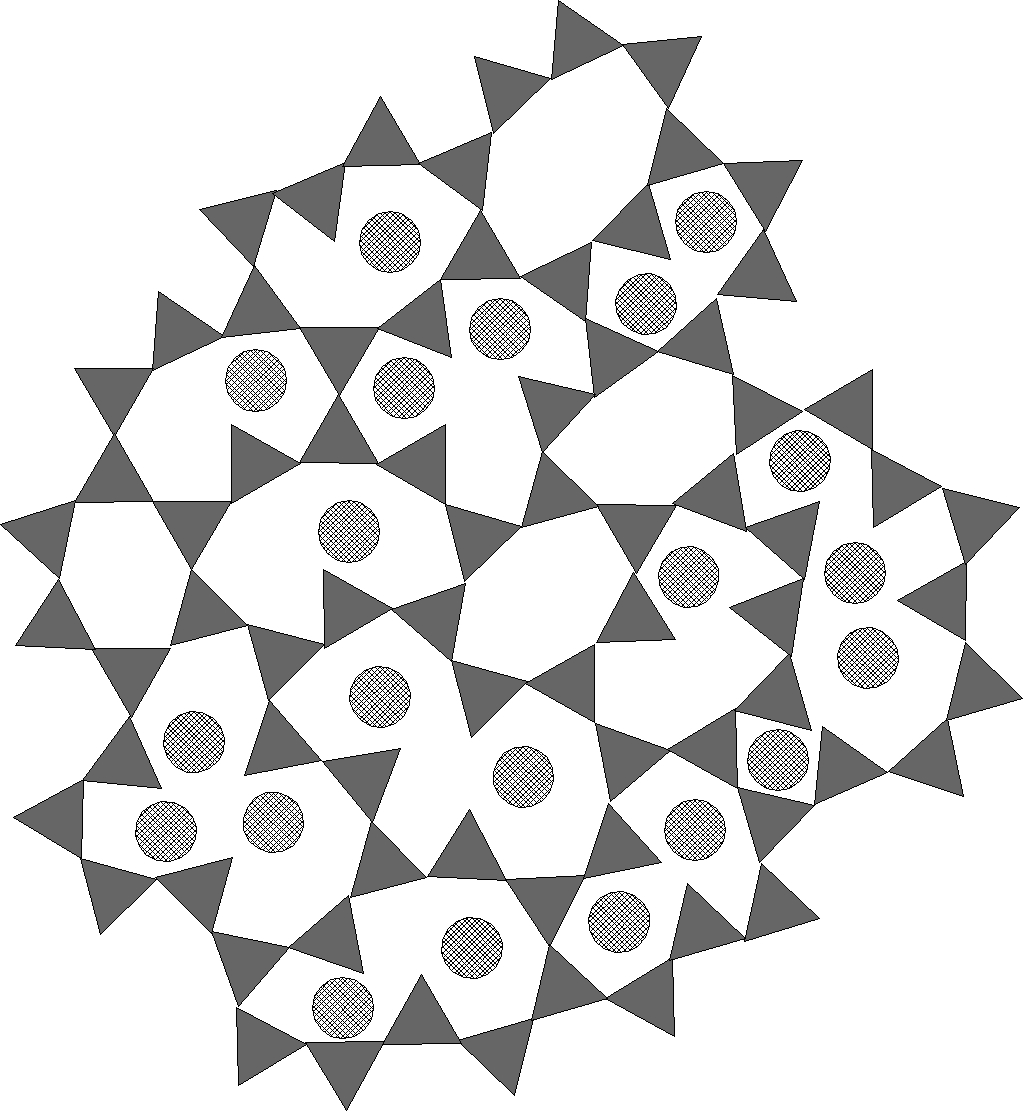
Figure 2 - silica glass with Na2O
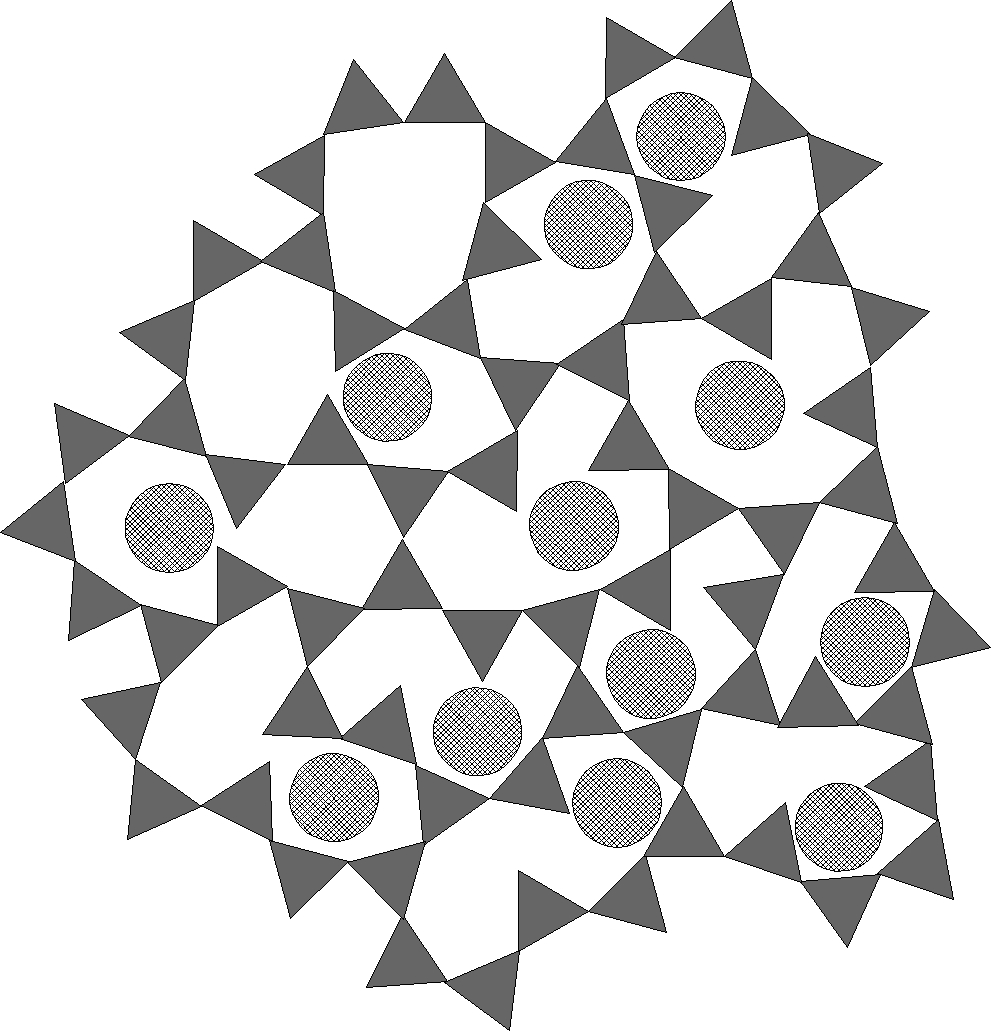
Figure 3 - silica glass with CaO
The first column elements in the Seger Formula for a ceramic glaze are the special sauce. Without these oxides there would be no pottery glazes. Silica by itself melts at to high a temperature for any ordinary kiln, and at that temperature, there are no clays that could hold their shape.
The bases alias network modifiers. do just exactly that, modify the network by breaking it up, making it more open, less connected, and more flexible. This partial dissconecting and the additional freedom, reducing the inhibitions if you wish, is the mechanism by which the glassy material can melt at a temperature which accomodates the making of pottery.
We show below in two-dimensional cartoons the change in structure that accompanies additions of the alkali metals, and calcium oxide to a glassy melt.
For reference, we show in figure 1 first a drawing of pure silica, rigid, all the internal triangle vertices are connected. In Figure 2 we show a silica glass containing alkali metal, Na2O, K2O or Li2O. Then in Figure 3 we show a silica glass containing an alkaline earth, CaO or MgO.
Note that the alkali metals occur almost always in pairs and are smaller, so fit more easily into the network. The alkaline earths occur singly, but are larger, requiring like a big bully, a larger opening in the network, or by their presence creating a larger disruption of the network.


