The role of Boron in Ceramic glazes
The three columns in the Seger Formula for a Ceramic Glaze:
Column three, the network formers. This column contains the essence, the ur building
material, the blocks, or in this case tetrahedra, of which the glaze is built.
that which makes it all possible, its first element silica.
Column one, the network modifiers, or if you prefer, network busters, forming
holes in the network, similar to runs in a stocking. This column contains the
magic sauce, the bases which enable the glaze to melt and fuse at a
temperature viable for pot and potter.
Column two, the amphoterics, the switch hitter, the play it either way
components. We have met Alumina which is a network former, yet tightens the
network, resulting in a more refractory also more viscous melt. This is
essential for the potter, enables the glaze to melt without flowing off the
pot.
Boron
Boron sits also in column two, it modifies the network without tearing holes in
it as the network modifiers do. Boron can sit in the network as silica does,
as a tetrahedron. The boron tetrahedra are slightly smaller than the silica
tetrahedra. Alternatively, drum roll here, the boron can form triangles.
Boron, on its ownsies, can form a glass, with a lower melting temperature than
any silica glass. This is a result of the flopyness inherent in stringing
together planar triangles in space. Mixing some boron triangles into a silica
melt, brings to the silica the flexibility of the boron glass. The result can
be fusion at a slightly lower temperature. The network doesn't loose its
connectivity however, so that glaze properties are altered without a change in
maturing temperature. The effect is often seen as a smoother, more lusterous
glaze which fits the body better, but matures at the same temperature.
As always, we show the boron tetrahedra as triangles in our two dimensional
cartoons, the boron triangle are projected onto the plane as line segments, of
the appropriate length, a bit shorter than the edges from the silica
tetrahedra.
For reference we show silica and an alkali silicate glass. The remaining
figures are of silica with tetrahedral boron, where the boron are shown as
slightly smaller hatched triangles to distinguish them from the silica. Next
is silica with the boron triangles projected onto lines, in its opening the
network role. Last, a drawing of silica with boron (in its triangular form,
shown here as edges) and alumina to show that the boron opens where the
alumina tightens, balancing each other.
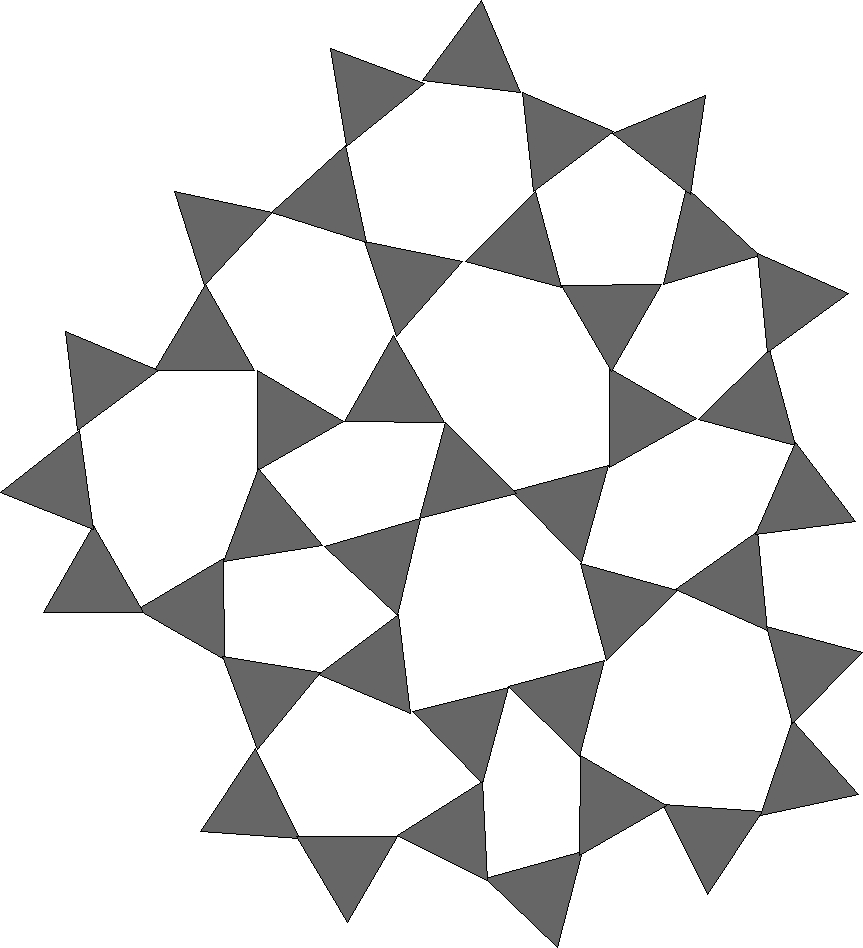
Figure 1 - Silica Glass
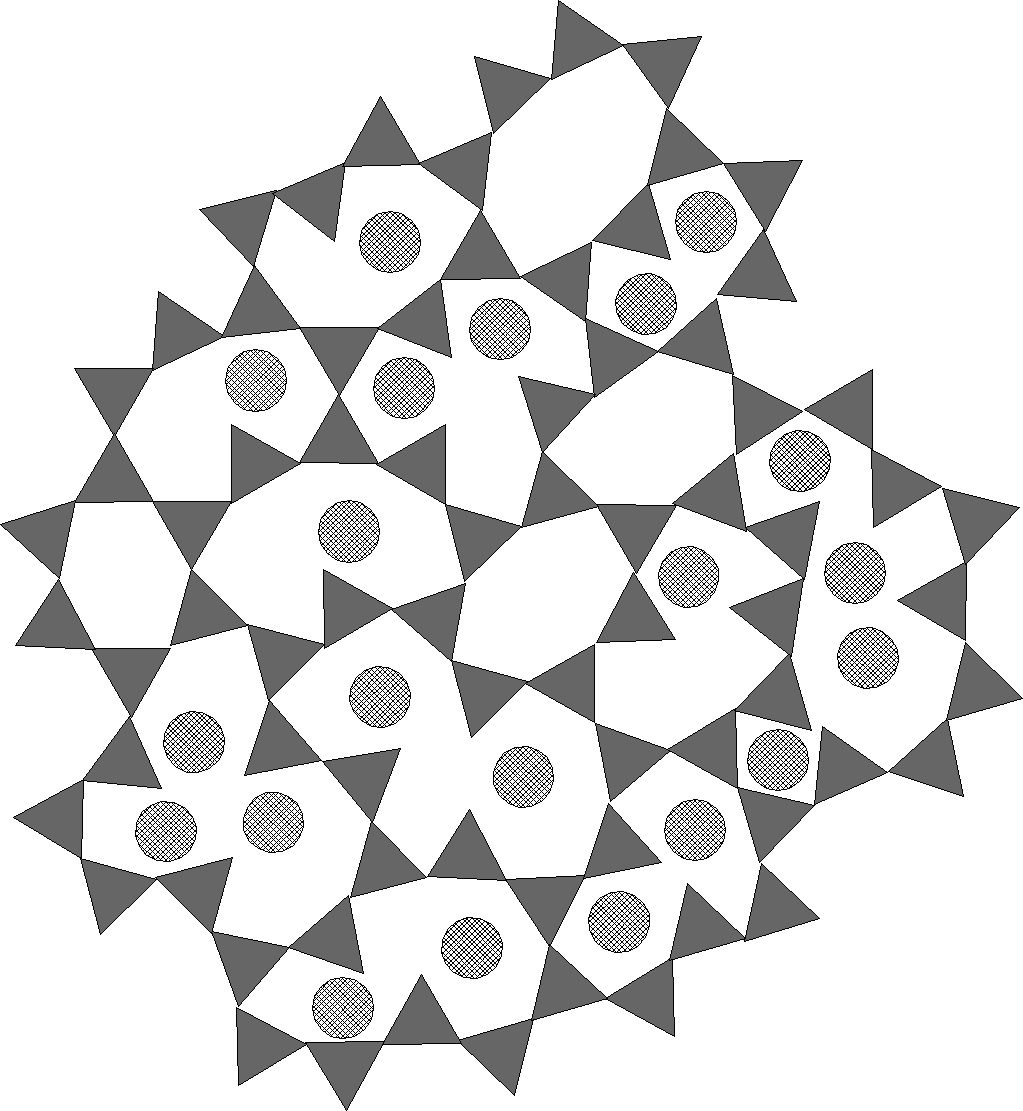
Figure 2 - silica glass with Na2O
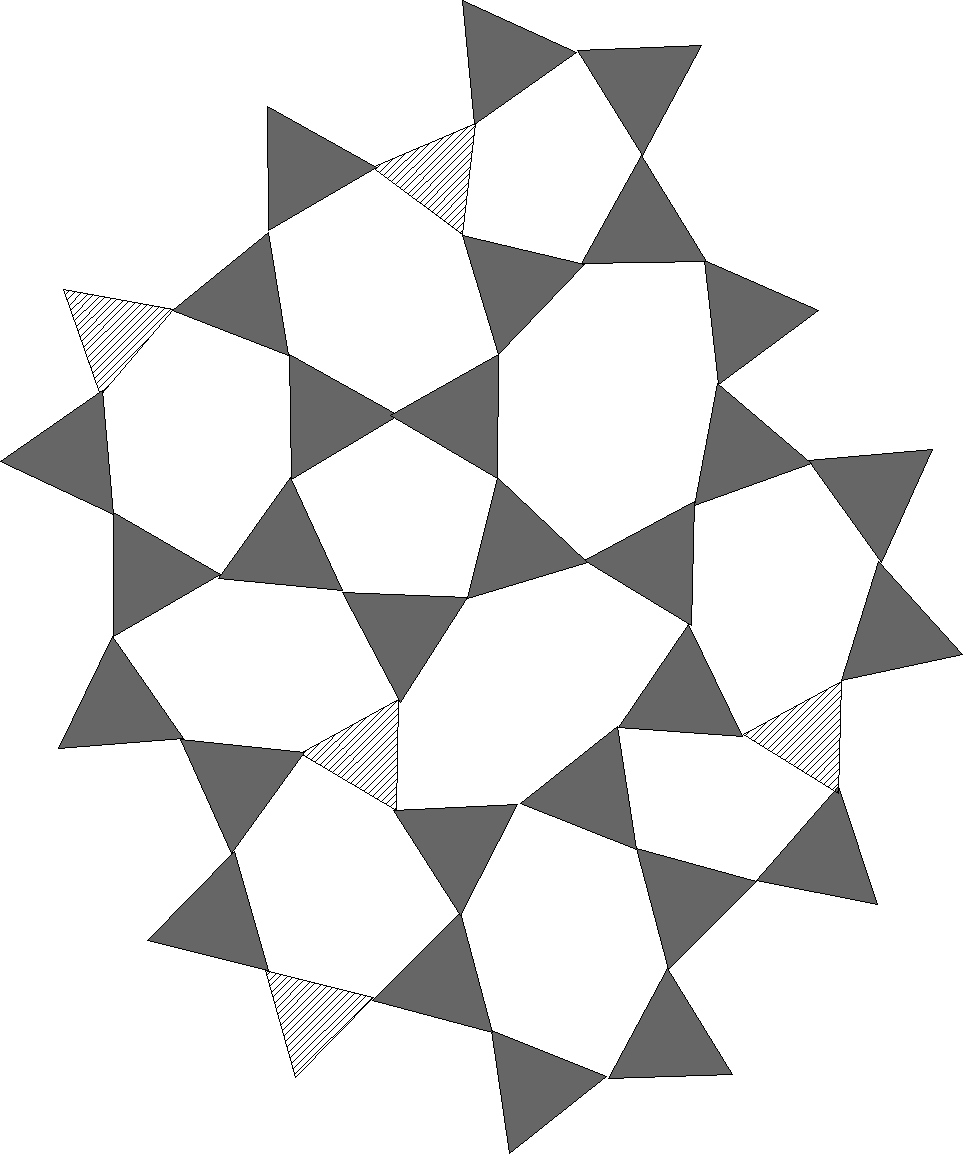
Figure 3 - silica with tetrahedral Boron
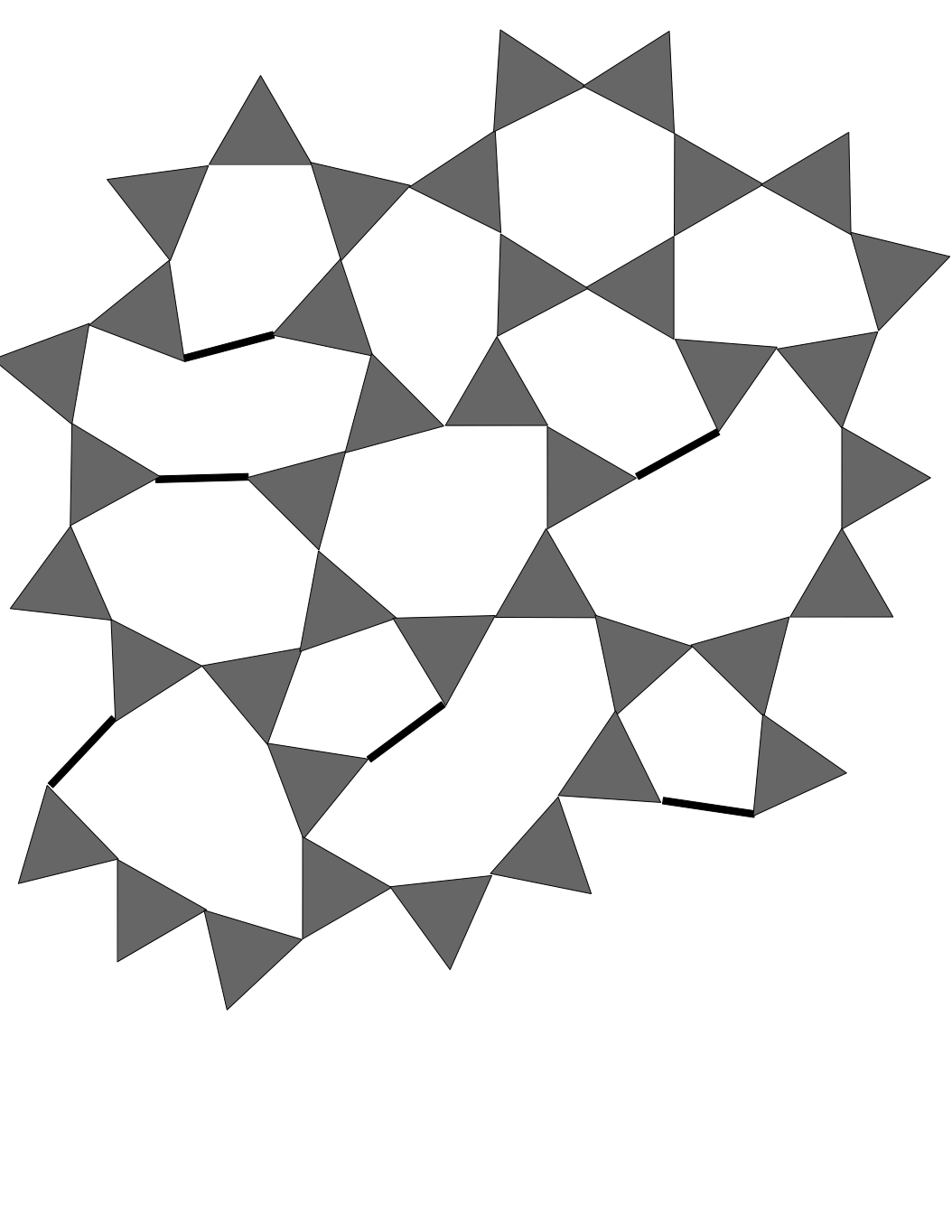
Figure 4 - silica with triangular Boron
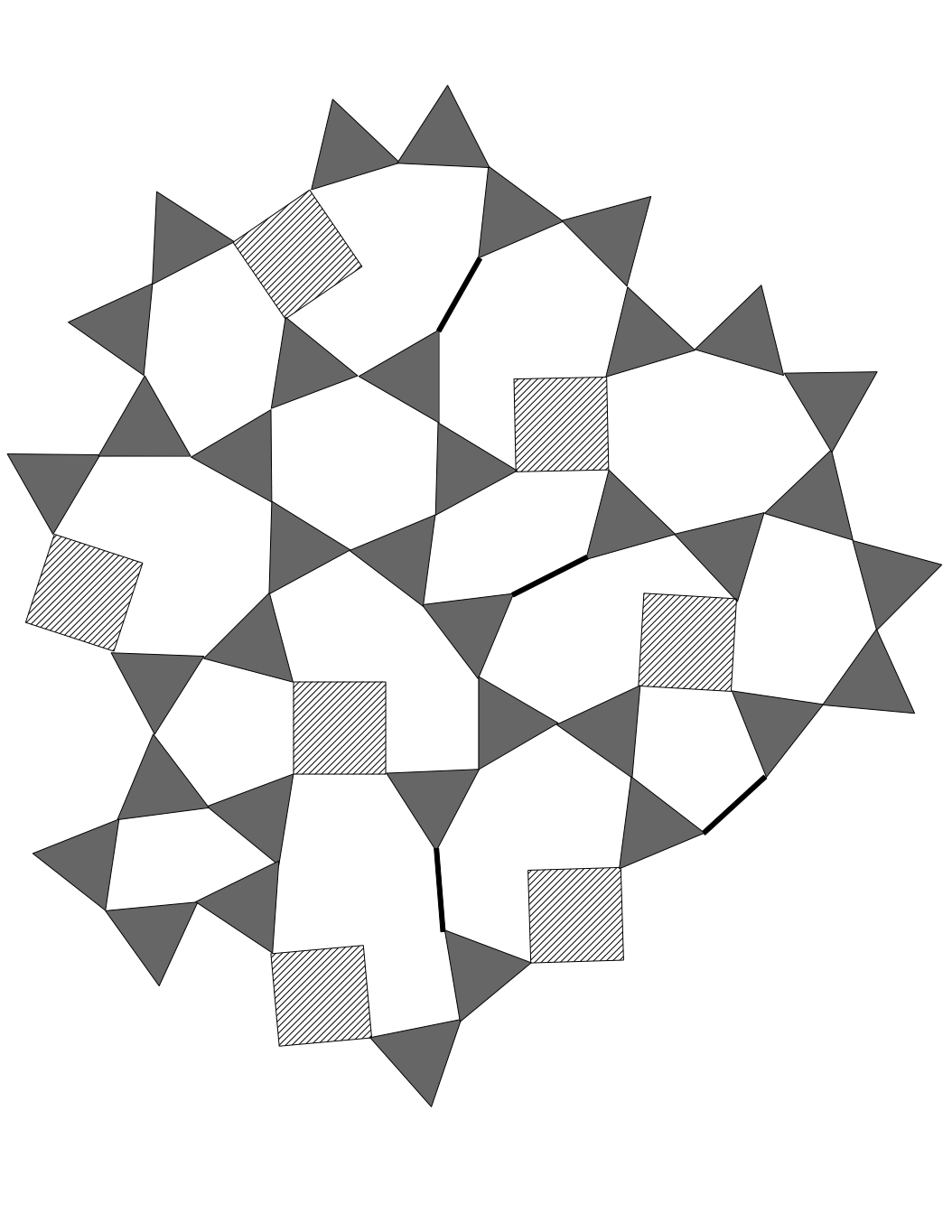
Figure 5 - Silica with Boron and Alumina
Home Page of Carol Marians




