variations on a saturated iron glaze
firing to cone 10 in oxidation
A half hour hold at 1850 deg F
slow downfire at 50 deg F an hour in the interval 1850 deg F to 1700 deg F
slow downfire at 25 deg F an hour in the interval 1700 deg F to 1650 deg F
One hour hold at 1650 deg F
Variations on a saturated iron glaze
Here are small variations of the glaze iron_8_Right_162_1Z_1 which has massed golden crystals and metallic luster.
The Ur glaze iron_8_R_162_1Z_1

We test nearby compositions, to see how rapidly its appearance changes, and to understand in which directions its appearance changes most rapidly.
The perterbations of each glaze from the Ur glaze (iron_8_Right_162_1Z_1) are:
increase or decrease Alumina |
increase Alumina and Silica |
increase silica |
increase or decrease alkali metals |
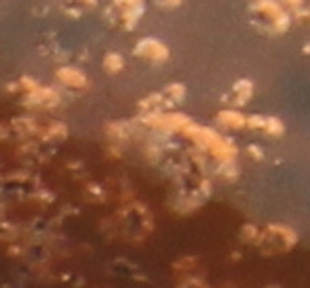
For this reason we show here a close up of a small cluster of metallic crystals to enable their identification in the pictures that follow.
small clusters of metallic crystals
We show close up images of the above variations of our Ur glaze.
glaze composition
The Ur glaze
is high in alkaline metals and in Li2O, with Al2O3 = .5, SiO2 = 3.15 and P2O5
= .04.
The variants are all high in alkali metals and Li2O. Al2O3 varies
from .44 to .6, and Silica from 2.8 to 3.5. P2O5 remains .04.
increased and decreased Alumina
The image on the left shows the result of a small increase in Alumina, on the
right a small decrease.
Neither looks quite like the original.
Surprisingly increasing alumina seemed to increase the abundance of metallic
crystals.
As that is contrary to expectation and the effect is small we
conclude that this glaze is relatively insensitive to small changes in alumina.
 |
 |
A leaf

This is a leaf print on satIron_ZG_0, the glaze with added alumina.
the
wash is custer feldspar with rutile added.
The painterly quality of this leaf print, in contrast to the image on the Ur
glaze shows a greater migration of properties than we'd suspected.
In the
upper left corner there is a small amount of a variant glaze visible.
increase of silica, and of both silica and alumina
The glaze on the left has a small addition of silica to the Ur glaze,
that
on the right a small addition of both silica and alumina.
Again we note
that contrary to intuition, increasing both silica and alumina seemed to
increase the dominance of metallic crystals.
Putting this together with
the above, and the smallness of the effect
we are tempted to think this
glaze varies slowly with increases of alumina and silica, together or
independantly.
 |
 |
increase or decrease of alkali metals
The glaze shown on the left has some alkaline earths replaced with alkali metals (K2O, Na2O, Li2O), the one on the right the reverse, some alkali metals replaced with alkaline earths.
Here we see the first substantial shift in properties, an increase in alkali metals gives a glaze with a dense coverage of metallic crystals.
decreasing the alkali metals removes visible metallic crystals.
 |
 |