variations on a saturated iron glaze
firing to cone 6 in oxidation
A half hour hold at 1850 deg F
slow downfire at 50 deg F an hour in the interval 1850 deg F to 1700 deg F
slow downfire at 25 deg F an hour in the interval 1700 deg F to 1650 deg F
One hour hold at 1650 deg F
Here are small variations of the glaze iron_8_Right_MgPSi which has massed golden crystals and metallic luster.
The Ur glaze iron_8_Right_MgPSi

The orange markings are the result of a reactive slip on the body beneath the glaze.
This glaze is troublesome,
it requires an exceedingly thick glaze application to show the crystals, yet
a slightly thicker application will
cause the glaze to shiver.
We test near by compositions,
to get a glaze which develops the crystals and doesn't
shiver.
The perterbations of each glaze from the Ur glaze (iron_8_Right_MgPsi) are:
increase or decrease Silica |
increase or decrease Alumina |
increase or decrease alkaline earths |
increase Na2O |
We show close up images of the above variations of our Ur glaze.
glaze composition
The Ur glaze
is high in alkaline metals and in Li2O, with Al2O3 = .42, SiO2 = 3.32 and P2O5
= .04.
The variants are all high in alkali metals and Li2O. Al2O3 varies
from .38 to .46, and Silica from 2.8 to 3.9. P2O5 remains .04.
increased and decreased Silica
The image on the top shows the result of a small increase in Silica, on the bottom a small decrease.
 |
 |
The pot on the left is ~3.5 inches high, the one on the right ~3 inches in diameter.
increased and decreased Alumina
The image on the top shows the result of a small increase in Alumina, on the bottom a small decrease.
 |
 |
These cylinder pots are ~4 inches in diameter.
increased and decreased Alkaline earths
The image on the top shows the result of a small increase in Alkaline earths, on the bottom a small decrease.
The glaze shown on the top has some alkali metals (K2O, Na2O, Li2O) replaced with alkaline earths, the one on the bottom the reverse, some alkaline earths replaced with alkali metals.
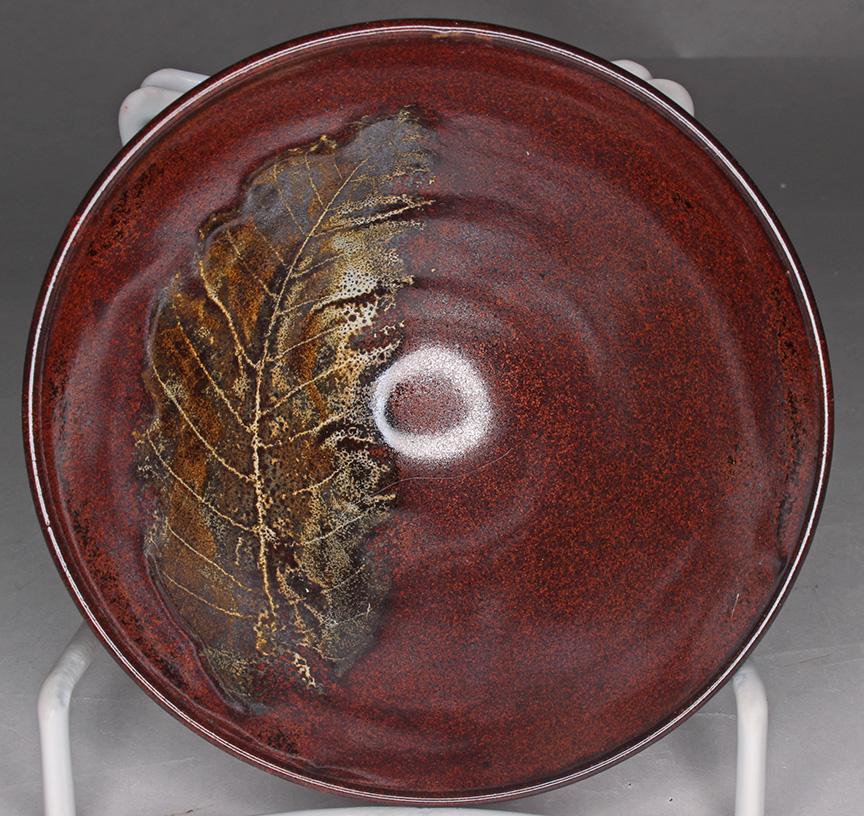 |
 |
These cone shaped rice bowls are are respectively 4.5 and 6.5 inches in diameter
We expect an increase in silica, alkaline earths or alumina to increase the
viscosity of the glaze, thus decrease crystal formation.
The dirction of
the shifts are therefore not a surprise.
However the Ur
glaze seems not to fall between any of these glazes pairs in appearance.
The color shifts are a surprise. the replacement of some K2O by Na2O lightened the
colors.
The replacement alkaline earths by alkali metals shifts the
colors toward yellow, which seemed also to be the effect of decreasing
alumina.
Finally a decrease in Silica, in addition to reducing the gloss,
shifts the colors lighter.
Increased Na2O
This glaze has some K2O replaced by Na2O

This cylindrical bowl is 6 inches in diameter