Color in glazes containing Chrome Oxide
cone 10 oxidation
The Downfire profile
A half hour hold at 1750 deg F
A three hour hold at 1700 deg F
slow downfire at 25 deg F an hour in the interval 1700 deg F to 1650 deg F
A one hour hold at 1650 deg F
Clay body is a grolleg porcelain from Tacoma Clay Art Center.
We last saw the two glazes paperWhite_ZO_1 and paperWhite_ZO_3 in:
We noted that this pair of glazes had a prominent bright green phase.
I
was curious to see the effect on the color of making these glazes matt without
changing the composition of the bases,
so I added sufficient Alumina
Hydroxide to produce an alumina matt. The fluxes (first column components of
the empirical formula) are unchanged,
and there is a substantial shift in
the colors.
paperWhite_ZO_3 with added alumina hydroxide in addition to the chrome oxide, is pinholed and likely underfired.
In a further exploration of the effect of the composition of the bases
(fluxes) on the color of a glaze containing .25 %
chrome oxide I tested two
glazes which differ only in the relative proportion of BaO and SrO.
In their respective empirical Formula's these two glaze have in common:
Alkali metals .44
Al2O3 .5
SiO2 3
The alkali metals are evenly split between K2O and Na2O
The alkaline earths are dominated by BaO and SrO:
paperWhite_ZP_0 has low BaO and high SrO with BaO .25, SrO .28.
paperWhite_ZP_1 has high BaO and low SrO with BaO .35, SrO .18.
The non test tiles are small bowls ~3 inches in diameter.

paperWhite_ZO_1 with 0.2 % Chrome oxide

paperWhite_ZO_1 with 0.2 % Chrome oxide and added alumina

paperWhite_ZO_3 with 0.2 % Chrome oxide

paperWhite_ZO_3 with 0.2 % Chrome oxide and added alumina
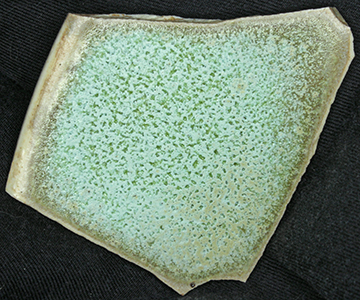
paperWhite_ZP_0 with 0.2 % Chrome oxide

paperWhite_ZP_1 with 0.2 % Chrome oxide
Commentary
Note that the addition of alumina to paperWhite_ZO_1 and to paperWhite_ZO_3 causes a striking color shift.
Equally, paperWhite_ZP_0 and paperWhite_ZP_1 show the effect of a change in balance between BaO and SrO, from turquoise to bright apple green.