underfired
The glaze cooper_404_1_PAl is underfired.
Several closeups of the surface are shown and, for context, a picture
of the pot.
There is a pink artifact which perhaps is the result of inhomogeneity in the
glaze mixture. I suspect it is the ZnO
150 deg F an hour to 250 deg F
400 deg F an hour to 1800 deg F
300 deg F an hour to 2050 deg F
120 deg F an hour to 2310 deg F with a hold of 20 minutes at 2310 deg F
300 deg F an hour to 1750 deg F then a half hour hold at 1750 deg F
300 deg F an hour to 1700 deg F then a Three hour hold at 1700 deg F
25 deg F an hour to 1650 deg F then a one hour hold at 1650 deg F
K2O 0.14
Al2O3 0.38
SiO2 2.38
molecular percent Silica 63%
Added 1.5% Nickel Oxide
The close-up pictures were taken with a digital microscope.
The coarse-grained microcrystalline structure of the surface shows this
glaze to be underfired. The pits that appear
The roll of glaze at the bottom of the pot, showing alumina picked up from the
shelf, shows this to be an exceedingly fluid melt.
Here is a composition which, while it didn't mature into a glaze (i.e. is
underfired), is more fluid than one would desire a glaze to be.
which is not fully mixed into the
glaze.
Close up Images of the surface


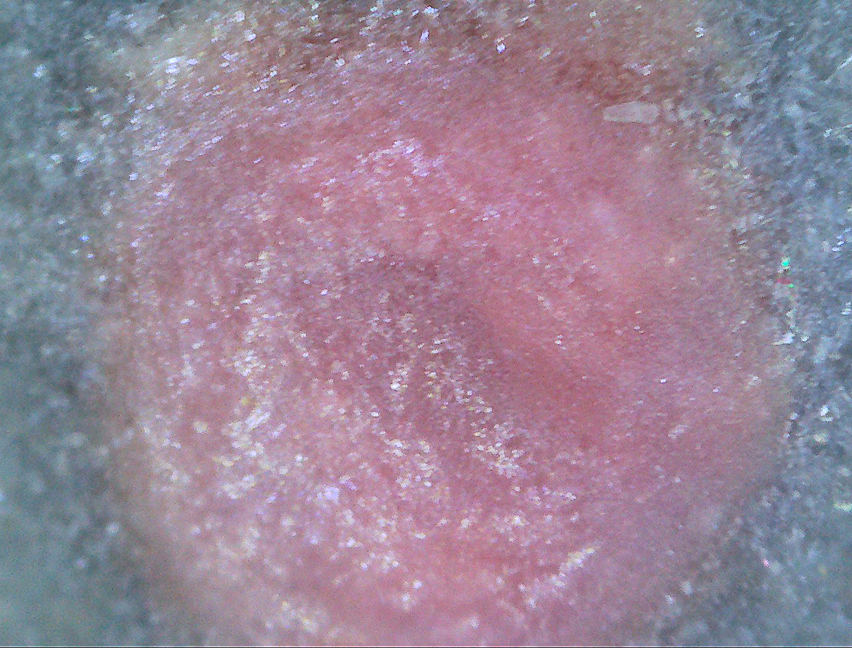
Image of the piece

inside

outside
bowl is is 4 inches in diameter
oxidation firing to cone 10 in an electric kiln
Firing profiles
Up Fire profile
Down Fire Profile
Clay body is a grolleg porcelain from Tacoma Clay Art Center.
glaze compositions
Empirical Formula cooper_404_1_PAl :
Na2O 0.22
CaO 0.02
MgO 0.01
BaO 0.22
ZnO 0.39
Remarks
to be pinholes are seen in the
enlargements as edged in micro-crystals. These are voids created by
continuation
of the chemical reactions that are part of forming the melt.
They are not the residue of escaping gas from an already melted glaze.